Unlocking the potential of biofuels via reaction pathways in van Krevelen diagrams†
Abstract
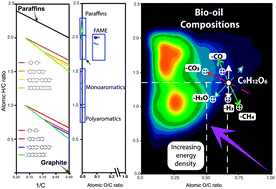
Production of fuels and targeted chemicals from biomass represents a current challenge. Pyrolysis of biomass generates liquid bio-oils but these are highly complex mixtures. In order to obtain the desired products, optimized reaction conditions are required and this, in turn, drives the need for a fundamental understanding of the complex reaction network. Bio-oils are a complex mixture of thousands of individual molecular compositions, with differing numbers of carbon, hydrogen, nitrogen, and oxygen atoms (c, h, n, and o, respectively). The compositional spaces of such complex mixtures with high oxygen contents are commonly plotted using van Krevelen diagrams, where the H/C versus O/C ratios are displayed. For a bio-oil to be effectively used in engines, further upgrading is necessary to drive the compositions towards low oxygen and high hydrogen content (thus, low O/C and high H/C values). Here, we propose reaction vectors in van Krevelen diagrams to outline the possible reaction routes that favour the production of molecules with increased energy density, using examples of bio-oils produced from citrus waste (lemon and orange peel) and olive pulp. When reactions such as the addition or loss of CO, CO2, CH4, and H2O occur, a displacement of the compositions of molecules in terms of H/C and O/C coordinates is observed. The direction and magnitude of the displacement along each axis in van Krevelen diagrams depends upon the specific reaction route and the elemental content of each molecule. As a consequence of the wide diversity of compositions, different reaction routes are suggested that include multi-step upgrading processes, including hydrogenation and the elimination of oxygen in the form of CO and CO2. The detailed molecular composition of the starting material, plotted in van Krevelen diagrams for visualization, paves the way for greater insight into potential reaction pathways for components within these highly complex mixtures. In turn, the equations proposed hold potential to inform future production strategies, increasing the energy density of bio-oils whilst also reducing the undesirable char formation.


 Please wait while we load your content...
Please wait while we load your content...