Ring-contraction of hantzsch esters and their derivatives to pyrroles via electrochemical extrusion of ethyl acetate out of aromatic rings†
Abstract
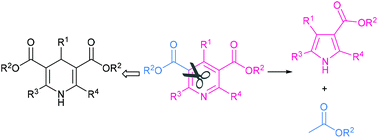
Electrochemical ring-contraction of HEs and theirs pyridine derivatives is developed to obtain polysubstituted pyrroles. This process provides an orthogonal utilization of Hantzsch esters for the well-documented application as side chain or hydrogen donors. The formal transformation shows an extrusion of ethyl acetate out of the pyridine ring in a single step. In addition to the novel transformation, we also discovered the Lewis acid's intermolecular control of regioselectivity during an intramolecular electrochemical process. The reaction provides a number of polysubstituted pyrroles that have never been accessed, including pharmaceutical intermediates and photoswitches. An unusual 4-electron continuous reduction drives the unprecedented anionic dearomatization/ring-contraction/rearomatization pathway.


 Please wait while we load your content...
Please wait while we load your content...