Sustainable electrochemical decarboxylative acetoxylation of aminoacids in batch and continuous flow†
Abstract
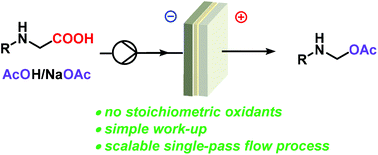
Introduction of acetoxy groups to organic molecules is important for the preparation of many active ingredients and synthetic intermediates. A commonly used and attractive strategy is the oxidative decarboxylation of aliphatic carboxylic acids, which entails the generation of a new C(sp3)–O bond. This reaction has been traditionally carried out using excess amounts of harmful lead(IV) acetate. A sustainable alternative to stoichiometric oxidants is the Hofer-Moest reaction, which relies on the 2-electron anodic oxidation of the carboxylic acid. However, examples showing electrochemical acetoxylation of amino acids are scarce. Herein we present a general and scalable procedure for the anodic decarboxylative acetoxylation of amino acids in batch and continuous flow mode. The procedure has been applied to the derivatization of several natural and synthetic amino acids, including key intermediates for the synthesis of active pharmaceutical ingredients. Good to excellent yields were obtained in all cases. Transfer of the process from batch to a continuous flow cell signficantly increased the reaction throughput and space–time yield, with excellent product yields obtained even in a single-pass. The sustainability of the electrochemical protocol has been examined by evaluating its green metrics. Comparison with the conventional method demonstrates that an electrochemical approach has a significant positive effect on the greenness of the process.


 Please wait while we load your content...
Please wait while we load your content...