Oxygen vacancy modulated interface chemistry: identifying iron(iv) in heterogeneous Fenton reaction†
Abstract
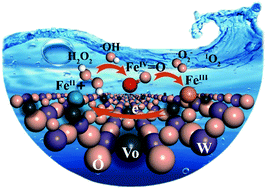
Introducing transition-metal oxides as co-catalysts into classical Fenton chemistry holds great promise for improving the recycling of iron species. However, the underlying chemistry that controls the generation and transformation of ferryl species (FeIV) during such heterogeneous Fenton reactions is not fully understood. Herein, we modulated oxygen-vacancy-enriched WO3−x and identified surface FeIV species using in situ spectroscopy and density functional theory calculations. Direct spectroscopic evidence shows that WO3−x caused the reaction of FeII with H2O2 to switch from the formation of FeIII complexes towards direct generation of FeIV. FeIV intermediates oxidize H2O2 to ˙O2−/1O2, accompanied by the production of FeIII. FeIII is reduced to FeII by the electrons localized in the t2g orbitals of WO3−x, stimulating the generation of ˙OH. This study opens a new chapter in the mechanistic understanding of FeIV formation and extends the development of co-catalysts via surface engineering in remediation techniques.


 Please wait while we load your content...
Please wait while we load your content...