Carbon–titanium dioxide (C/TiO2) nanofiber composites for chemical oxidation of emerging organic contaminants in reactive filtration applications†
Abstract
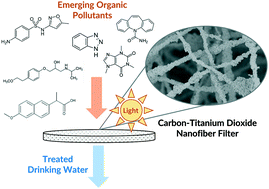
The recalcitrance of some emerging organic contaminants through conventional water treatment systems may necessitate advanced technologies that use highly reactive, non-specific hydroxyl radicals. Here, polyacrylonitrile (PAN) nanofibers with embedded titanium dioxide (TiO2) nanoparticles were synthesized via electrospinning and subsequently carbonized to produce mechanically stable carbon/TiO2 (C/TiO2) nanofiber composite filters. Nanofiber composites were optimized for reactivity in flow through treatment systems by varying their mass loading of TiO2, adding phthalic acid (PTA) as a dispersing agent for nanoparticles in electrospinning sol gels, comparing different types of commercially available TiO2 nanoparticles (Aeroxide® P25 and 5 nm anatase nanoparticles) and through functionalization with gold (Au/TiO2) as a co-catalyst. High bulk and surface TiO2 concentrations correspond with enhanced nanofiber reactivity, while PTA as a dispersant makes it possible to fabricate materials at very high P25 loadings (∼80% wt%). The optimal composite formulation (50 wt% P25 with 2.5 wt% PTA) combining high reactivity and material stability was then tested across a range of variables relevant to filtration applications including filter thickness (300–1800 μm), permeate flux (from 540–2700 L m−2 h), incident light energy (UV-254 and simulated sunlight), flow configuration (dead-end and cross-flow filtration), presence of potentially interfering co-solutes (dissolved organic matter and carbonate alkalinity), and across a suite of eight organic micropollutants (atrazine, benzotriazole, caffeine, carbamazepine, DEET, metoprolol, naproxen, and sulfamethoxazole). During cross-flow recirculation under UV-irradiation, 300 μm thick filters (30 mg total mass) produced micropollutant half-lives ∼45 min, with 40–90% removal (from an initial 0.5 μM concentration) in a single pass through the filter. The initial reaction rate coefficients of micropollutant transformation did not clearly correlate with reported second order rate coefficients for reaction with hydroxyl radical (kOH), implying that processes other than reaction with photogenerated hydroxyl radical (e.g., surface sorption) may control the overall rate of transformation. The materials developed herein represent a promising next-generation filtration technology that integrates photocatalytic activity in a robust platform for nanomaterial-enabled water treatment.


 Please wait while we load your content...
Please wait while we load your content...
