Moisture-resistant Nb-based fluoride K2NbF7:Mn4+ and oxyfluoride phosphor K3(NbOF5)(HF2):Mn4+: synthesis, improved luminescence performance and application in warm white LEDs†
Abstract
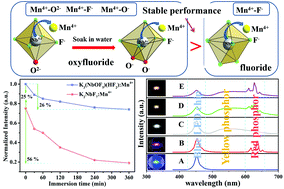
Herein, high-efficiency Nb-based oxyfluoride K3(NbOF5)(HF2):Mn4+ and fluoride K2NbF7:Mn4+ phosphors were successfully synthesized using different amounts of HF acid solutions by a simple co-precipitation method. XRD, SEM and EDS were used to characterize the crystal structure, morphology and elemental composition of the phosphors. The emission spectra, excitation spectra and luminescence decay curves were used to study the luminescence characteristics of the samples. The thermal stability of the phosphors was tested and the mechanism of temperature quenching was discussed. Meanwhile, the moisture resistance and application of the phosphors were investigated in detail. The results show that the K3(NbOF5)(HF2):Mn4+ phosphor has stronger luminous intensity, lower color temperature, and better moisture resistance compared with the K2NbF7:Mn4+ phosphor. The correlated color temperature (CCT) and color rendering index (CRI) of warm white LEDs can be significantly improved by using the K3(NbOF5)(HF2):Mn4+ (CCT = 3430 K, CRI = 87.3) phosphor as a red light component. So the K3(NbOF5)(HF2):Mn4+ phosphor has broader application prospect in the field of warm white LEDs.


 Please wait while we load your content...
Please wait while we load your content...