Noninnocence of the deprotonated 1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine bridge in diruthenium frameworks – a function of co-ligands†
Abstract
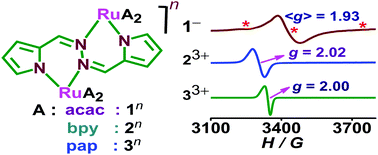
The article deals with the sensitive electronic forms in accessible redox states of structurally and spectroscopically authenticated deprotonated 1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine (H2LR, R = H) or 1,2-bis((3,5-dimethyl-1H-pyrrol-2-yl)methylene)hydrazine (H2LR, R = Me), a BODIPY analogue bridged diruthenium complex as a function of varying ancillary ligands. It involved rac-(acac)2RuIII(μ-LR 2−)RuIII(acac)21a, R = H; 1b, R = Me (S = 1, acac = acetylacetonate), rac-[(bpy)2RuII(μ-L2−)RuII(bpy)2](ClO4)2 [2](ClO4)2 (S = 0, bpy = 2,2′-bipyridine) and diastereomeric [(pap)2RuII(μ-L2−)RuII(pap)2](ClO4)2meso-[3a](ClO4)2/rac-[3b](ClO4)2 (S = 0, pap = phenylazopyridine). The crystal structure established the linkage of the conjugated –C5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N2–N3
N2–N3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C6– central unit with the two terminal deprotonated pyrrole units of coordinated L2−. The bridging L2− in 1a, 1b, [2](ClO4)2, [3b](ClO4)2 and [3a](ClO4)2 was slightly twisted and planar with torsional angles of 41.54°, 42.91°, 37.38°, 35.33° and 0°, respectively, with regard to the central N2–N3 bond. The extent of twisting of the bridge followed an inverse relationship with the Ru⋯Ru separation: 4.935/4.934 Å 1a/1b < 5.141 Å [2](ClO4)2 < 5.201 Å [3b](ClO4)2 < 5.351 Å [3a](ClO4)2. This is also attributed to the intermolecular π⋯π/CH⋯π interactions between the nearby aromatic rings of L and bpy or pap in [2](ClO4)2 or [3](ClO4)2, respectively. The multiple redox steps of the complexes varied appreciably based on the σ-donating (acac) and π-acidic (bpy, pap) characteristics of the ancillary ligands. Experimental (structure, EPR) and theoretical (DFT) evaluation pertaining to the electronic forms of 1n, 2n and 3n demonstrated the preferential involvement of L based frontier orbitals in electron transfer processes even in combination with the redox facile ruthenium ion. This in turn highlighted its redox non-innocent feature as in the case of well-documented metal coordinated quinonoid, formazanate, diimine (bpy), azo (pap) and β-diketiminate functions.
C6– central unit with the two terminal deprotonated pyrrole units of coordinated L2−. The bridging L2− in 1a, 1b, [2](ClO4)2, [3b](ClO4)2 and [3a](ClO4)2 was slightly twisted and planar with torsional angles of 41.54°, 42.91°, 37.38°, 35.33° and 0°, respectively, with regard to the central N2–N3 bond. The extent of twisting of the bridge followed an inverse relationship with the Ru⋯Ru separation: 4.935/4.934 Å 1a/1b < 5.141 Å [2](ClO4)2 < 5.201 Å [3b](ClO4)2 < 5.351 Å [3a](ClO4)2. This is also attributed to the intermolecular π⋯π/CH⋯π interactions between the nearby aromatic rings of L and bpy or pap in [2](ClO4)2 or [3](ClO4)2, respectively. The multiple redox steps of the complexes varied appreciably based on the σ-donating (acac) and π-acidic (bpy, pap) characteristics of the ancillary ligands. Experimental (structure, EPR) and theoretical (DFT) evaluation pertaining to the electronic forms of 1n, 2n and 3n demonstrated the preferential involvement of L based frontier orbitals in electron transfer processes even in combination with the redox facile ruthenium ion. This in turn highlighted its redox non-innocent feature as in the case of well-documented metal coordinated quinonoid, formazanate, diimine (bpy), azo (pap) and β-diketiminate functions.


 Please wait while we load your content...
Please wait while we load your content...