Low temperature synthesis of barium oxynitridosilicates using BaCN2 and SiO2†
Abstract
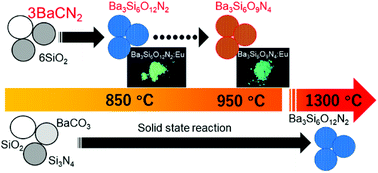
Barium oxynitridosilicates, Ba3Si6O12N2 and Ba3Si6O9N4, were obtained from a mixture of BaCN2 and SiO2 at 800 °C, which is several hundred degrees lower than the temperature required in solid state reactions using BaCO3, SiO2 and Si3N4. The low-temperature formation mechanism was investigated by thermogravimetry analysis in conjunction with gas chromatography and mass spectroscopy. The phase ratio between the oxynitridosilicates was controlled by tuning the reaction temperature, duration, and atmosphere. Almost single-phase Ba3Si6O12N2 was obtained by reaction at 800 °C for 15 h under a N2 atmosphere, but the product changed to Ba3Si6O9N4 after 50 h at 800 °C or by heating at 950 °C for 15 h. The photoluminescence properties of Eu-doped products obtained at 800 °C using a mixture of BaCN2 : Eu and SiO2 were investigated.


 Please wait while we load your content...
Please wait while we load your content...