Temperature dependence of desolvation effects in hydrogen-bonded spin crossover complexes†
Abstract
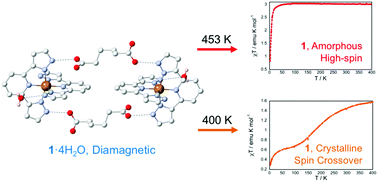
The synthesis, crystal structure and (photo)magnetic properties of the anhydrous spin crossover salt of formula [Fe(bpp)2](C6H8O4) (1) (bpp = 2,6-bis(pyrazol-3-yl)pyridine; C6H8O4 = adipate dianion), obtained by desolvation at 400 K of the original tetrahydrate in a single-crystal-to-single-crystal (SC–SC) transformation, are reported. This work offers a comparison between this compound and the previously reported hydrated material ([Fe(bpp)2](C6H8O4)·4H2O, 1·4H2O), highlighting the significance of the thermal conditions used in the dehydration-rehydration processes. In both compounds, a hydrogen-bonded network between iron(II) complexes and adipate anions is observed. The original tetrahydrate (1·4H2O) is low-spin and desolvation at 450 K triggers a low-spin (LS) to high-spin (HS) transition to an amorphous phase that remains stable over the whole temperature range of study. Surprisingly, the dehydrated compound at 400 K (1) keeps the crystallinity, undergoes a partial spin crossover (T1/2 = 180 K) and a quantitative LS to HS photomagnetic conversion at low temperatures, with a T(LIESST) value of 61 K.


 Please wait while we load your content...
Please wait while we load your content...