Steering the methanol steam reforming reactivity of intermetallic Cu–In compounds by redox activation: stability vs. formation of an intermetallic compound–oxide interface†
Abstract
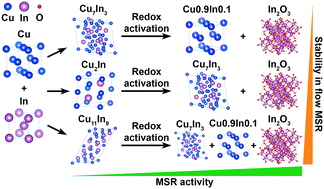
To compare the inherent methanol steam reforming properties of intermetallic compounds and a corresponding intermetallic compound–oxide interface, we selected the Cu–In system as a model to correlate the stability limits, self-activation and redox activation properties with the catalytic performance. Three distinct intermetallic Cu–In compounds – Cu7In3, Cu2In and Cu11In9 – were studied both in an untreated and redox-activated state resulting from alternating oxidation–reduction cycles. The stability of all studied intermetallic compounds during methanol steam reforming (MSR) operation is essentially independent of the initial stoichiometry and all accordingly resist substantial structural changes. The inherent activity under batch MSR conditions is highest for Cu2In, corroborating the results of a Cu2In/In2O3 sample accessed through reactive metal–support interaction. Under flow MSR operation, Cu7In3 displays considerable deactivation, while Cu2In and Cu11In9 feature stable performance at simultaneously high CO2 selectivity. The missing significant self-activation is most evident in the operando thermogravimetric experiments, where no oxidation is detected for any of the intermetallic compounds. In situ X-ray diffraction allowed us to monitor the partial decomposition and redox activation of the Cu–In intermetallic compounds into Cu0.9In0.1/In2O3 (from Cu7In3), Cu7In3/In2O3 (from Cu2In) and Cu7In3/Cu0.9In0.1/In2O3 (from Cu11In9) interfaces with superior MSR performance compared to the untreated samples. Although the catalytic profiles appear surprisingly similar, the latter interface with the highest indium content exhibits the least deactivation, which we explain by formation of stabilizing In2O3 patches under MSR conditions.


 Please wait while we load your content...
Please wait while we load your content...