Synergetic effect of Cu active sites and oxygen vacancies in Cu/CeO2–ZrO2 for the water–gas shift reaction†
Abstract
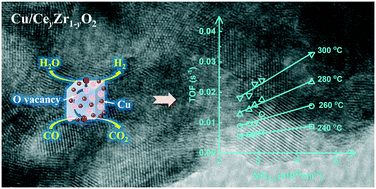
A series of Cu/CeO2–ZrO2 catalysts with different Ce/(Ce + Zr) molar ratios and Cu loadings were prepared by a simple citrate sol–gel method, characterized by various techniques, and evaluated for water–gas shift (WGS) under H2-rich and low H2O/CO molar ratio conditions of the sorption enhanced WGS process. It was shown that the oxygen vacancy concentration and the Cu dispersion were important structural factors affecting the catalytic activity of Cu/CeO2–ZrO2, but neither of them alone was capable of correlating with the turnover frequency (TOF) for WGS based on kinetic analysis. By contrast, a positive linear correlation was established between the TOF and the ratio of oxygen vacancy concentration to Cu dispersion, demonstrating the synergetic effect of Cu active sites and oxygen vacancies for WGS. The stability of Cu/CeO2–ZrO2 was found to be dependent on the crystal structure of CeO2–ZrO2 solid solution, and the cubic to tetragonal phase transformation of CeO2–ZrO2 led to a decreased stability. Among all Cu/CeO2–ZrO2 investigated, the catalyst with a Ce/(Ce + Zr) molar ratio of 0.6 and a Cu loading of 50 wt% showed the highest activity and stability under 20% H2, 40% CO and 40% steam in a gas hourly space velocity of 20 000 h−1 at 350 °C, with the CO conversion slightly varying from 77% to 75% over 60 h on stream and meanwhile 100% selectivities to H2 and CO2.


 Please wait while we load your content...
Please wait while we load your content...