Hierarchically porous magnetic Fe3O4/Fe-MOF used as an effective platform for enzyme immobilization: a kinetic and thermodynamic study of structure–activity†
Abstract
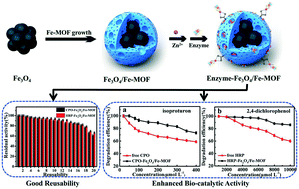
Enzymes are efficient catalysts offering much more competitive processes than conventional chemical reactions. However, the lack of stability and reusability is a key issue on the use of enzymes in industrial applications. These drawbacks can generally be overcome by immobilization of the enzyme on a solid support. In this work, a Fe3O4/Fe-MOF core–shell with hierarchical pore sizes was prepared by growing Fe-MOF around Fe3O4 nanoparticles via solvothermal synthesis. Fe3O4/Fe-MOF integrates magnetic characteristics and hierarchical porous structure for supporting chloroperoxidase (CPO) or horseradish peroxidase (HRP). Immobilized CPO/HRP exhibited significantly enhanced stability against temperature stress and facilitated reuse. For example, HRP-Fe3O4/Fe-MOF can retain 94.1% activity after 10 reuses. Moreover, CPO/HRP-Fe3O4/Fe-MOFs were very efficient in the degradation of organic toxins, isoproturon or 2,4-dichlorophenol in simulated wastewater with a complete degradation within 15 min. The excellent performance of CPO/HRP-Fe3O4/Fe-MOF was investigated by kinetic and thermodynamic analysis. The results indicated that the diffusion resistance of substrates was reduced compared with that in a bulk solution because they were initially concentrated close to enzymes on the hierarchically porous support. The specificity and binding affinity of the immobilized enzymes are enhanced compared with the free enzymes.


 Please wait while we load your content...
Please wait while we load your content...