Recent applications of asymmetric organocatalytic annulation reactions in natural product synthesis
Abstract
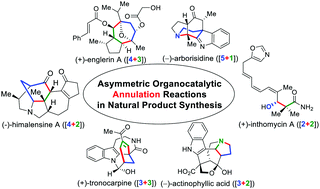
The past two decades have witnessed remarkable growth of asymmetric organocatalysis, which is now a firmly established synthetic tool, serving as a powerful platform for the production of chiral molecules. Ring structures are ubiquitous in organic compounds, and, in the context of natural product synthesis, strategic construction of ring motifs is often crucial, fundamentally impacting the eventual fate of the whole synthetic plan. In this review, we provide a comprehensive and updated summary of asymmetric organocatalytic annulation reactions; in particular, the application of these annulation strategies in natural product synthesis will be highlighted.


 Please wait while we load your content...
Please wait while we load your content...