CH2 + O2: reaction mechanism, biradical and zwitterionic character, and formation of CH2OO, the simplest Criegee intermediate†
Abstract
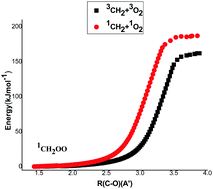
The singlet and triplet potential surfaces for the title reaction were investigated using the CBS-QB3 level of theory. The wave functions for some species exhibited multireference character and required the CASPT2/6-31+G(d,p) and CASPT2/aug-cc-pVTZ levels of theory to obtain accurate relative energies. A Natural Bond Orbital Analysis showed that triplet 3CH2OO (the simplest Criegee intermediate) and 3CH2O2 (dioxirane) have mostly polar biradical character, while singlet 1CH2OO has some zwitterionic character and a planar structure. Canonical variational transition state theory (CVTST) and master equation simulations were used to analyze the reaction system. CVTST predicts that the rate constant for reaction of 1CH2 + 3O2 is more than ten times as fast as the reaction of 3CH2 (X3B1) + 3O2 and the ratio remains almost independent of temperature from 900 K to 3000 K. The master equation simulations predict that at low pressures the 1CH2O + 3O product set is dominant at all temperatures and the primary yield of OH radicals is negligible below 600 K, due to competition with other primary reactions in this complex system.


 Please wait while we load your content...
Please wait while we load your content...