Two-bond 13C–13C spin-coupling constants in saccharides: dependencies on exocyclic hydroxyl group conformation†
Abstract
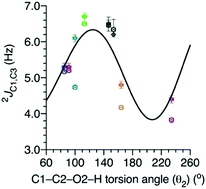
Seven doubly 13C-labeled isotopomers of methyl β-D-glucopyranoside, methyl β-D-xylopyranoside, methyl β-D-galactopyranoside, methyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside and methyl β-D-galactopyranosyl-(1→4)-β-D-xylopyranoside were prepared, crystallized, and studied by single-crystal X-ray crystallography and solid-state 13C NMR spectroscopy to determine experimentally the dependence of 2JC1,C3 values in aldopyranosyl rings on the C1–C2–O2–H torsion angle, θ2, involving the C2 carbon of the C1–C2–C3 coupling pathway. Using X-ray crystal structures to determine θ2 in crystalline samples and by selecting compounds that exhibit a relatively wide range of θ2 values in the crystalline state, 2JC1,C3 values measured in crystalline samples were plotted against θ2 and the resulting plot compared to that obtained from density functional theory (DFT) calculations. For θ2 values ranging from ∼90° to ∼240°, very good agreement was observed between the experimental and theoretical plots, providing strong validation of DFT-calculated spin-coupling dependencies on exocyclic C–O bond conformation involving the central carbon of geminal C–C–C coupling pathways. These findings provide new experimental evidence supporting the use of 2JCCC values as non-conventional spin-coupling constraints in MA′AT conformational modeling of saccharides in solution, and the use of NMR spin-couplings not involving coupled hydroxyl hydrogens as indirect probes of C–O bond conformation. Solvomorphism was observed in crystalline βGal-(1→4)-βGlcOCH3 wherein the previously-reported methanol solvate form was found to spontaneously convert to a monohydrate upon air-drying, leading to small but discernible conformational changes in, and a new crystalline form of, this disaccharide.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
