Exploring the binding mechanism of positive allosteric modulators in human metabotropic glutamate receptor 2 using molecular dynamics simulations†
Abstract
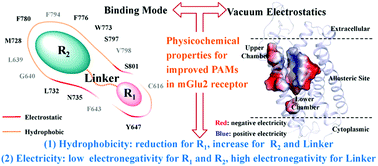
Positive allosteric modulators (PAMs) of human metabotropic glutamate receptor 2 (hmGlu2) are well-known in the treatment of psychiatric disorders for their higher selectivity and lower tolerance risk. A variety of PAMs have been reported over the last decade and two compounds were in Phase II clinical trials for schizophrenia and anxiety. These trials were discontinued on account of the unsatisfactory therapeutic efficacy, but PAMs were explored as novel treatments for addiction and epilepsy. Thus, it is still important to explore novel hmGlu2 PAMs in the near future. Nowadays, the challenges in optimizing drug potency and improving scaffold diversity for PAMs are the noncomprehensive character analyses of multiple scaffolds; the exploration of the binding modes of PAMs in the allosteric binding site have been proposed to reduce this difficulty. However, there has been no comprehensive research about the binding profiles of PAMs in the hmGlu2 receptor. To address this issue, this work explores the binding characters of eight PAMs representing five chemical series by multiple computational methods. As a result, the shared binding modes of the eight studied PAMs interacting with 15 residues in the allosteric binding site were defined. In addition, the reduced hydrophobicity with low electronegativity of R1, increased hydrophobicity with low negative electron density of R2 and the electronegativity of the linker were identified as indicators that regulate the affinity of PAMs. This finding agrees well with the physicochemical properties of reported multiple series PAMs. This comprehensive work sheds additional light on the binding mechanism and physicochemical regularity underlining PAMs affinity and could be further utilized as a structural and energetic blueprint for discovering and assessing novel PAMs for hmGlu2.


 Please wait while we load your content...
Please wait while we load your content...