Transmembrane penetration mechanism of cyclic pollutants inspected by molecular dynamics and metadynamics: the case of morpholine, phenol, 1,4-dioxane and oxane
Abstract
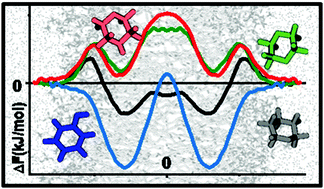
The presence of industrially produced chemicals in water is often not monitored, while their passive transport and accumulation can cause serious damage in living cells. Molecular dynamics simulations are an effective way to understand the mechanism of the action of these pollutants. In this paper, the passive membrane transport of 1,4-dioxane, phenol, oxane and morpholine was investigated and analyzed thoroughly from structural and energetic points of view. Free energy profiles for pollutant and water penetration into the bilayer were obtained from well-tempered metadynamics (WT-MD) simulations and a mass density-based approach. It was found that all four investigated compounds can penetrate biological membranes and affect the free energy profile of water penetration. Out of the investigated species, oxane has the thermodynamically most preferred position in the bilayer center, leading to a lower free energy barrier of water molecules by 3 kJ mol−1, resulting in 5 times more water molecules in the bilayer center. The concentration dependence of free energy was tested at two different phenol concentrations using WT-MD, and it was found that the higher phenol concentration lowers the main barrier by 3 kJ mol−1. Density-based free energy calculations were found to reproduce the results of WT-MD within the limits of chemical accuracy.


 Please wait while we load your content...
Please wait while we load your content...