Oxidation of HOSO˙ by Cl˙: a new source of SO2 in the atmosphere?†
Abstract
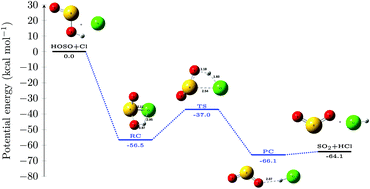
In the present work, we have studied the formation of SO2 in the atmosphere from the oxidation of HOSO˙ by Cl˙ at the CCSD(T)/aug-cc-pV(+d)TZ//MP2/aug-cc-pV(+d)TZ level of theory. The present work reveals that the title reaction is a barrierless reaction that proceeds through a stable intermediate sulfurochloridous acid having a stabilization energy of ∼−56.5 kcal mol−1. The rate constant values within the temperature range of 213–400 K indicate that the rate of HOSO˙ + Cl˙ = SO2 + HCl reaction does not change much with the change in temperature. Besides, the reaction was also found to be insensitive towards pressure change. Interestingly, the relative rate of HOSO˙ + Cl˙ reaction with respect to HOSO˙ + OH˙ reaction indicates that HOSO˙ + Cl˙ is always much slower than HOSO˙ + OH˙ reaction, within the temperature range of 213–400 K.


 Please wait while we load your content...
Please wait while we load your content...