Characterization of two photon excited fragment spectroscopy (TPEFS) for HNO3 detection in gas-phase kinetic experiments†
Abstract
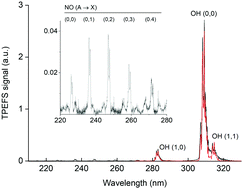
We have developed and tested two-photon excited fragment spectroscopy (TPEFS) for detecting HNO3 in pulsed laser photolysis kinetic experiments. Dispersed (220–330 nm) and time-dependent emission at (310 ± 5) nm following the 193 nm excitation of HNO3 in N2, air and He was recorded and analysed to characterise the OH(A2Σ) and NO(A2Σ+) electronic excited states involved. The limit of detection for HNO3 using TPEFS was ∼5 × 109 molecule cm−3 (at 60 torr N2 and 180 μs integration time). Detection of HNO3 using the emission at (310 ± 5 nm) was orders of magnitude more sensitive than detection of NO and NO2, especially in the presence of O2 which quenches NO(A2Σ+) more efficiently than OH(A2Σ). While H2O2 (and possibly HO2) could also be detected by 193 nm TPEFS, the relative sensitivity (compared to HNO3) was very low. The viability of real-time TPEFS detection of HNO3 using emission at (310 ± 5) nm was demonstrated by monitoring HNO3 formation in the reaction of OH + NO2 and deriving the rate coefficient, k2. The value of k2 obtained at 293 K and pressures of 50–200 torr is entirely consistent with that obtained by simultaneously measuring the OH decay and is in very good agreement with the most recent literature values.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...