Spectral tuning of chlorophylls in proteins – electrostatics vs. ring deformation†
Abstract
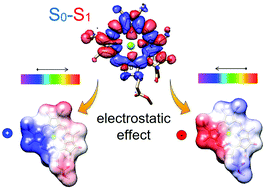
In photosynthetic complexes, tuning of chlorophyll light-absorption spectra by the protein environment is crucial to their efficiency and robustness. Recombinant type II water soluble chlorophyll-binding proteins from Brassicaceae (WSCPs) are useful for studying spectral tuning mechanisms due to their symmetric homotetramer structure, and the ability to rigorously modify the chlorophyll's protein surroundings. Our previous comparison of the crystal structures of two WSCP homologues suggested that protein-induced chlorophyll ring deformation is the predominant spectral tuning mechanism. Here, we implement a more rigorous analysis based on hybrid quantum mechanics and molecular mechanics calculations to quantify the relative contributions of geometrical and electrostatic factors to the absorption spectra of WSCP–chlorophyll complexes. We show that when considering conformational dynamics, geometry distortions such as chlorophyll ring deformation accounts for about one-third of the spectral shift, whereas the direct polarization of the electron density accounts for the remaining two-thirds. From a practical perspective, protein electrostatics is easier to manipulate than chlorophyll conformations, thus, it may be more readily implemented in designing artificial protein–chlorophyll complexes.


 Please wait while we load your content...
Please wait while we load your content...