Efficient and chemoselective hydrogenation of aldehydes catalyzed by well-defined PN3–pincer manganese(ii) catalyst precursors: an application in furfural conversion†
Abstract
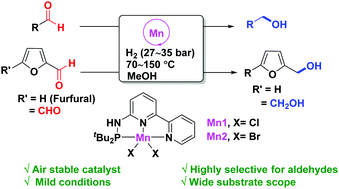
Well-defined and air-stable PN3–pincer manganese(II) complexes were synthesized and used for the hydrogenation of aldehydes into alcohols under mild conditions using MeOH as a solvent. This protocol is applicable for a wide range of aldehydes containing various functional groups. Importantly, α,β-unsaturated aldehydes, including ynals, are hydrogenated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond/C
C double bond/C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond intact. Our methodology was demonstrated for the conversion of biomass derived feedstocks such as furfural and 5-formylfurfural to furfuryl alcohol and 5-(hydroxymethyl)furfuryl alcohol respectively.
C triple bond intact. Our methodology was demonstrated for the conversion of biomass derived feedstocks such as furfural and 5-formylfurfural to furfuryl alcohol and 5-(hydroxymethyl)furfuryl alcohol respectively.


 Please wait while we load your content...
Please wait while we load your content...