Counting molecules in nano test tubes: a method for determining the activation parameters of thermally driven reactions through direct imaging†
Abstract
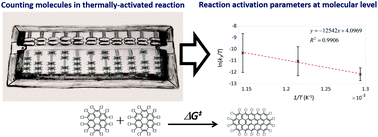
A methodology for measuring activation parameters of a thermally driven chemical reaction by direct imaging and counting reactant molecules has been developed. The method combines the use of single walled carbon nanotubes (SWNTs) as a nano test tube, transmission electron microscopy (TEM) as an imaging tool, and a heating protocol that decouples the effect of the electron beam from the thermal activation. Polycyclic aromatic perchlorocoronene molecules are stable within SWNTs at room temperature, allowing imaging of individual molecules before and after each heating cycle between 500–600 °C. Polymerisation reaction rates can be determined at different temperatures simply by counting the number of molecules, resulting in an enthalpy of activation of 104 kJ mol−1 and very large entropic contributions to the Gibbs free energy of activation. This experimental methodology provides a link between reactions at the single-molecule level and macroscopic chemical kinetics parameters, through filming the chemical reaction in direct space.


 Please wait while we load your content...
Please wait while we load your content...