Palladium-catalyzed stereoselective domino arylation–acylation: an entry to chiral tetrahydrofluorenone scaffolds†
Abstract
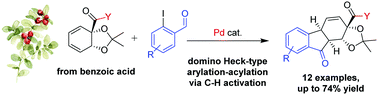
A palladium-catalyzed domino arylation-cyclization of biocatalytically derived cyclic 1,3-dienes is demonstrated. The reaction introduces a high degree of structural complexity in a single step, giving access to tricyclic tetrahydrofluorenones with full regio- and stereoselectivity. The transformation proceeds through a novel acylation-terminated Heck-type sequence, and quantum chemical calculations indicate that C–H activation is involved in the terminating acylation step.


 Please wait while we load your content...
Please wait while we load your content...