Aromaticity in catalysis: metal ligand cooperation via ligand dearomatization and rearomatization†
Abstract
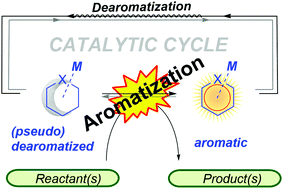
Unlike the conventional model of transition metal catalysis, ligands in metal–ligand cooperative (or bifunctional) catalysis are involved in the substrate activations. Such processes have offered unique mechanistic understandings and led to new concepts for the catalyst design. In particular, unprecedented activities were discovered when the ligand could undergo dearomatization–rearomatization reactions during the catalytic cycle. Aromatization can provide an extra driving force to thermodynamics; consequently, it brings a new perspective to ligand platform design for catalysis. While numerous applications were demonstrated, the influences of changing ligand aromatic properties were often overlooked. In this article, representative ligand systems will be highlighted and a comparison between the Milstein and the Huang pincer systems will be discussed to provide theoretical and conceptual insights.


 Please wait while we load your content...
Please wait while we load your content...