A hybrid ocular delivery system of cyclosporine-A comprising nanomicelle-laden polymeric inserts with improved efficacy and tolerability
Abstract
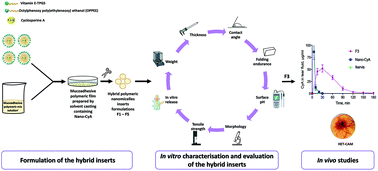
We report on hybrid nanomicelle–polymer inserts for improved delivery of cyclosporine A (CyA) to the surface of the eye. Hybrid inserts containing a nanomicellar formulation were prepared by the solvent casting method; their characteristics, in vitro release of CyA, eye irritation potential, nanomicelle distribution inside the insert, and in vivo pharmacokinetics of the most promising solid formulation (F3) were investigated. Nanomicelles capable of accommodating a therapeutically relevant amount of CyA (57.22 ± 5.90–68.52 ± 1.4 μg) were incorporated into five different polymeric formulations (F1–F5). The developed inserts displayed promising characteristics (size, weight, surface pH, and contact angle) that fulfill ocular tolerability requirements. Considering the technological properties and CyA in vitro release, F3 and F5 were the most promising formulations. SEM analysis suggested the F3 formulation as the potential prototype for CyA ocular delivery. The F3 formulation (CyA: 60.08 ± 2.85 μg) did not induce conjunctival irritation when HET-CAM assay was performed and was hence considered suitable for further study in a rabbit eye. The AUC value for CyA loaded in the F3 insert was about 2-fold greater than that obtained with the Ikervis® used as a control formulation. F3 produced a significant reduction (of about 7-folds) in the rate of CyA elimination from the tear fluid relative to Ikervis® and about 4-fold greater reduction than Nano-CyA (p = 0.0187). The ability of F3 to delay the elimination of the drug from the precorneal area is particularly desirable when treating dry eye syndrome. Furthermore, F3 did not induce ocular discomfort, a typical characteristic of solid ocular inserts, including commercially available ones.


 Please wait while we load your content...
Please wait while we load your content...