Phenylboronic acid-based core–shell drug delivery platform clasping 1,3-dicarbonyl compounds by a coordinate interaction†
Abstract
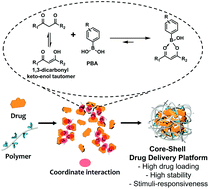
Along with the successful commercialization of chemotherapeutics, such as doxorubicin and paclitaxel, numerous natural compounds have been investigated for clinical applications. Recently, curcumin (CUR), a natural compound with various therapeutic effects, has attracted attention for cancer immunotherapy. Most chemotherapeutics, however, have poor water solubility due to their hydrophobicity, which makes them less suited to biomedical applications; CUR is no exception because of its low bioavailability and extremely high hydrophobicity. In the present study, we developed an easy but effective strategy using the interaction between the 1,3-dicarbonyl groups of drugs and phenylboronic acid (PBA) to solubilize hydrophobic drugs. First, we verified the coordinate interaction between 1,3-dicarbonyl and PBA using 3,5-heptanedione as a model compound, followed by CUR as a model drug. A PBA-grafted hydrophilic polymer was used to form a nanoconstruct by coordination bonding with CUR, which then made direct administration of the nanoparticles possible. The nanoconstruct exhibited remarkable loading capability, uniform size, colloidal stability, and pH-responsive drug release, attributed to the formation of core–shell nanoconstructs by coordinate interaction. The therapeutic nanoconstructs successfully showed both chemotherapeutic and anti-PD-L1 anticancer effects in cellular and animal models. Furthermore, we demonstrated the applicability of this technique to other 1,3-dicarbonyl compounds. Overall, our findings suggest a facile, but expandable strategy by applying the coordinate interaction between 1,3-dicarbonyl and PBA, which enables high drug loading and stimuli-responsive drug release.

 Please wait while we load your content...
Please wait while we load your content...