Co-assembly of curcumin and a cystine bridged peptide to construct tumor-responsive nano-micelles for efficient chemotherapy†
Abstract
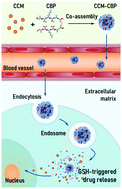
The effective uptake and release of hydrophobic antitumor drugs in cancer cells is a practical challenge for tumor chemotherapy. Many methods were developed to conquer it through modifying drug molecules with hydrophilic groups, or fabricating nanodrugs based on hydrophilic materials. In recent years, peptides have attracted significant interest as part of a promising platform for fabricating nanodrugs due to their low cytotoxicity, favorable variability and self-assembly property. In this study, a cystine bridged peptide (CBP) was designed to co-assemble with a hydrophobic antitumor drug curcumin (CCM), to form a tumor-responsive nanodrug. The hydrophilicity of the peptide promotes the water-dispersity of nanodrugs, and the disulfide bond in cystine, which is cleavable by glutathione (GSH), was involved considering the overexpressed GSH in tumor microenvironments. In vitro and in vivo tests on cervical cancer cells revealed that the obtained nanodrug can rapidly dissociate at tumor sites and inhibit the tumor growth with limited side effects on healthy tissues.


 Please wait while we load your content...
Please wait while we load your content...