Electron affinity regulation on ultrathin manganese oxide nanosheets toward ultra-stable pseudocapacitance†
Abstract
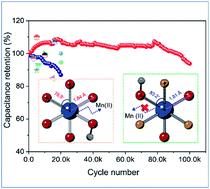
Although manganese oxide (MnO2) has long been considered as a promising electrode material for pseudocapacitors due to its high theoretical capacity and large potential window, its rapid capacity fading severely impedes its further large-scale applications. Herein, an electron affinity regulation strategy is developed to inhibit the dissolution of Mn2+ during the charging and discharging process. Remarkably, fluoride (F−) substituted MnO2 (MnOF) nanosheets exhibit an exceptionally high durability (no obvious degradation after 100 000 cycles even at a high scan rate of 200 mV s−1) along with an enhanced capacitance in an aqueous (Na2SO4) electrolyte, which is superior to that of all the reported MnOx electrodes and comparable to that of carbon-based electrodes. DFT calculations and X-ray fine structure characterization reveal that the non-equilibrium F substitution in MnO2 induces the enhanced energy barrier (ΔG) of the Mn(III) disproportionation reaction and greatly stabilizes the Mn–O bond, which are the key in boosting cycling life.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...