Elucidation of the high-voltage phase in the layered sodium ion battery cathode material P3–Na0.5Ni0.25Mn0.75O2†
Abstract
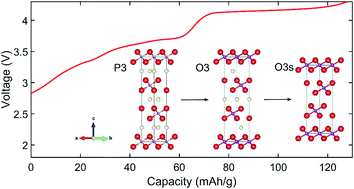
The P3-type layered oxide Na0.5Ni0.25Mn0.75O2 is a promising manganese-rich positive electrode (cathode) material for sodium ion batteries, with a high working voltage of 4.2–2.5 V vs. Na+/Na and a high capacity of over 130 mA h g−1 when cycled at 10 mA g−1. However, its structural evolution during battery cycling – specifically, the nature of the high-voltage phase above 4 V – has never been fully understood, which has hindered efforts to rationally modify and improve its performance. In this work we use in situ neutron diffraction to show that the phase above 4 V is a modification of the intermediate O3 phase from which all sodium has been removed, and which consequently has a dramatically shorter interlayer distance. We label this fully Na-depleted phase O3s, such that the phase evolution with increasing voltage is P3 → O3 → O3s. Having elucidated its structure, we used first-principles calculations of the electronic structure as a function of sodium content to show that reversible oxygen redox plays a key role in the electrochemical activity of this O3s phase above 4 V. We also calculated the energies of oxygen/transition metal vacancies and found that the O3s phase should be relatively stable against their formation. The results will guide future research aimed at understanding and stabilizing the O3s phase, in order to improve the performance and cycling stability of this material in sodium ion batteries.


 Please wait while we load your content...
Please wait while we load your content...