Anionic redox reactions and structural degradation in a cation-disordered rock-salt Li1.2Ti0.4Mn0.4O2 cathode material revealed by solid-state NMR and EPR†
Abstract
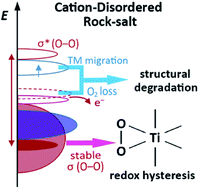
The burgeoning Li-rich cation-disordered rock-salt (DRX) materials have great potential to serve as the cathodes for rechargeable lithium ion batteries. They generally possess high capacities thanks to the participation of both cationic and anionic redox reactions. However, most DRX materials are subject to a large irreversible capacity loss during the first cycle and an undesirable voltage hysteresis between charge and discharge. In this work, solid-state NMR spectroscopy is employed to resolve the manifold local environments in Li1.2Ti0.4Mn0.4O2 as a model sample, and electron paramagnetic resonance (EPR) spectroscopy is used to investigate its electronic properties. Very broad resonances are observed in the 7Li and 17O NMR spectra. The paramagnetic and diamagnetic Li environments are distinguished, and the loss of the diamagnetic Li signal is observed after the first cycle. The degradation mechanism involved with oxygen loss and transition metal migration is discussed. The formation of the (O2)n− species and electron holes on oxygen at high voltage is suggested by EPR, and the voltage hysteresis is attributed to the difficulty in reducing the stable O–O bonds. These findings may enrich the understanding of DRX materials to better design high-performance DRX cathodes.


 Please wait while we load your content...
Please wait while we load your content...