A case of chain propagation: α-aminoalkyl radicals as initiators for aryl radical chemistry†
Abstract
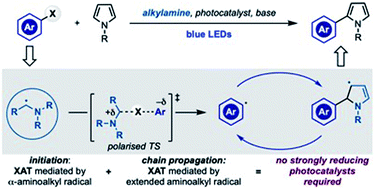
The generation of aryl radicals from the corresponding halides by redox chemistry is generally considered a difficult task due to their highly negative reduction potentials. Here we demonstrate that α-aminoalkyl radicals can be used as both initiators and chain-carriers for the radical coupling of aryl halides with pyrrole derivatives, a transformation often employed to evaluate new highly reducing photocatalysts. This mode of reactivity obviates for the use of strong reducing species and was also competent in the formation of sp2 C–P bonds. Mechanistic studies have delineated some of the key features operating that trigger aryl radical generation and also propagate the chain process.
- This article is part of the themed collections: Sustainable synthesis and catalysis – Chemical Science symposium collection and Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...