Et3SiH + KOtBu provide multiple reactive intermediates that compete in the reactions and rearrangements of benzylnitriles and indolenines†
Abstract
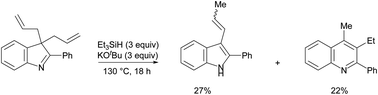
The combination of potassium tert-butoxide and triethylsilane is unusual because it generates multiple different types of reactive intermediates simultaneously that provide access to (i) silyl radical reactions, (ii) hydrogen atom transfer reactions to closed shell molecules and to radicals, (iii) electron transfer reductions and (iv) hydride ion chemistry, giving scope for unprecedented outcomes. Until now, reactions with this reagent pair have generally been explained by reference to one of the intermediates, but we now highlight the interplay and competition between them.


 Please wait while we load your content...
Please wait while we load your content...