Phosphoryl- and phosphonium-bridged viologens as stable two- and three-electron acceptors for organic electrodes†
Abstract
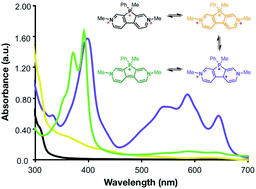
Low molecular weight organic molecules that can accept multiple electrons at high reduction potentials are sought after as electrode materials for high-energy sustainable batteries. To date their synthesis has been difficult, and organic scaffolds for electron donors significantly outnumber electron acceptors. Herein, we report the synthesis and electronic properties of two highly electron-deficient phosphaviologen derivatives from a phosphorus-bridged 4,4'-bipyridine and characterize their electrochemical properties. Phosphaviologen sulfide (PVS) and P-methyl phosphaviologen (PVM) accept two and three electrons at high reduction potentials, respectively. PVM can reversibly accept three electrons between 3–3.6 V vs. Li/Li+ with an equivalent molecular weight of 102 g (mol−1 e−) (262 mA h g−1), making it a promising scaffold for sustainable organic electrode materials having high specific energy densities.


 Please wait while we load your content...
Please wait while we load your content...