Dehydrating agent effect on the synthesis of dimethyl carbonate (DMC) directly from methanol and carbon dioxide
Abstract
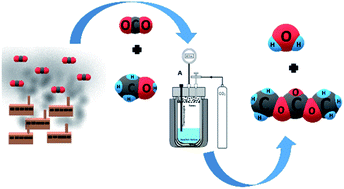
CO2 emissions and global warming have increased with the growth of the world economy and industrialization. Direct synthesis of dimethyl carbonate (DMC) from CO2 and methanol (CH3OH) has been considered a promising route from a green chemistry point of view due to global warming mitigation by CO2 emission reduction. However, DMC yield, when obtained by direct synthesis, is limited due to unfavorable thermodynamics and catalyst deactivation by water formation in the reaction process. This problem motivated us to investigate the effect of dehydration on DMC production by direct synthesis. Herein, different dehydrating agents (2,2-dimethoxypropane, sodium sulfate, magnesium oxide and butylene oxide) were combined with molecular sieves to remove the water and minimize the reverse reaction. A new reactor presenting a compartment to accommodate molecular sieves in the gas phase was developed as well. The chemical/product analysis was carried out by gas chromatography and the results were used to calculate methanol conversion and DMC selectivity. The highest methanol conversion value was found for the combination of molecular sieves in the gas phase with 2,2-dimethoxypropane in the reaction liquid phase (methanol conversion = 48.6% and 88% selectivity). The results showed that dehydration systems may promote increased yield in direct DMC synthesis under mild conditions. The dehydration systems tested in this work exhibited excellent conversion and yield as compared to other reported studies.


 Please wait while we load your content...
Please wait while we load your content...