Effect of polyvinylpyrrolidone (PVP) on palladium catalysts for direct synthesis of hydrogen peroxide from hydrogen and oxygen†
Abstract
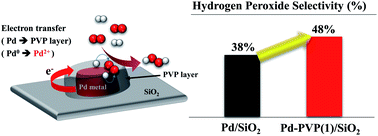
When synthesizing nanoparticles in the liquid phase, polymeric materials (mainly polyvinylpyrrolidone, PVP) are applied as capping and/or stabilizing agents. The polymer layer on the nanoparticles must likely be removed since it blocks the active sites of the catalyst and inhibits mass transfer of the reactants. However, we have found that the polymer can have a positive effect on the direct synthesis of hydrogen peroxide. By testing Pd/SiO2 catalysts with different amounts of PVP, it was revealed that an adequate amount of PVP resulted in a higher rate of hydrogen peroxide production (1001 mmolH2O2 gPd−1 h−1) than pristine Pd/SiO2 did (750 mmolH2O2 gPd−1 h−1), unlike other PVP added Pd/SiO2 catalysts containing excess PVP (less than 652 mmolH2O2 gPd−1 h−1). The effect of PVP on the catalysts was examined by transmission electron microscopy, Fourier transform infrared spectroscopy, CO chemisorption, thermogravimetric analysis, and X-ray photoelectron spectroscopy. For the catalysts containing PVP, the oxidation state of the palladium 3d shifted to high binding energy due to electron transfer from Pd to the PVP molecules. Consequently, the presence of PVP on the catalysts inhibited oxygen dissociation and decomposition of the produced hydrogen peroxide, resulting in a high selectivity and high production rate of hydrogen peroxide.


 Please wait while we load your content...
Please wait while we load your content...