Regioselective N1- and N2-heterocycloalkylation of N1-sulfonyl-1,2,3-triazoles†
Abstract
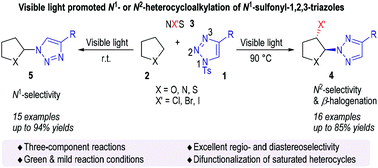
Considering the high synthetic value of N1- and N2-substituted compounds, we developed a tuneable and visible-light-driven three-component reaction between N1-sulfonyl-1,2,3-triazoles, saturated heterocycles, and N-bromosuccinimide to form N1- or N2-heterocycloalkylated 1,2,3-triazoles that are not easily accessible by conventional strategies. The visible-light-driven reaction generally proceeds smoothly and with excellent regioselectivity. In particular, the α-N2-1,2,3-triazole-β-halogenation of saturated heterocycles (formal C–C single bond difunctionalization) provided the corresponding products with excellent stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...