LiOtBu-promoted stereoselective deconjugation of α,β-unsaturated diesters probed using density functional theory†
Abstract
We report the isomerisation of α,β-unsaturated diesters to thermodynamically less favourable β,γ-unsaturated carbonyl compounds in the absence of strongly basic anhydrous or photochemical conditions. Stereoselective deconjugative isomerisation provides E-selective β,γ-unsaturated diesters via an enolate intermediate generated in situ from α,β-unsaturated diesters with high yield and stereoselectivity. Based on density functional theory calculations, the origin of the E-selective deconjugation pathway from conjugated compounds has been elucidated.
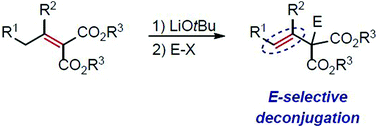


 Please wait while we load your content...
Please wait while we load your content...