Kinetic resolution of 2H-azirines via Cu(i)-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides†
Abstract
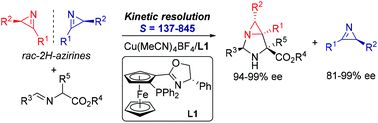
A highly efficient kinetic resolution of racemic 2H-azirines has been achieved by catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. With an (S,Sp)-Ph-Phosferrox/Cu(MeCN)4BF4 complex as the catalyst, optically pure 2H-azirines were obtained with excellent enantioselectivities (up to 99% ee) and high resolution efficiency (S factors up to 845). Meanwhile, this process provided a direct approach to synthesize structurally novel 1,3-diazabicyclo[3.1.0]hexane derivatives with excellent enantioselectivities (94–99% ee).


 Please wait while we load your content...
Please wait while we load your content...