Visible-light mediated oxidative ring expansion of anellated cyclopropanes to fused endoperoxides with antimalarial activity†
Abstract
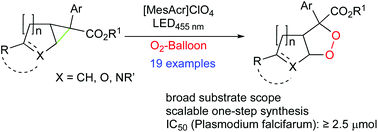
A visible light mediated ring expansion of readily available carbo- and heterocyclic anellated cyclopropanes by molecular oxygen at ambient pressure has been developed. Tolerating a variety of functional groups, the reaction yields fused 1,2-dioxolanes, which were tested for antimalarial activity given their close analogy to the active principle of approved drugs such as artemisinin.


 Please wait while we load your content...
Please wait while we load your content...
