One-pot access to 2-amino-3-arylbenzofurans: direct entry to polyheterocyclic chemical space†
Abstract
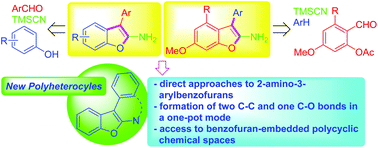
As a means to make new benzofuran-embedded polycyclic structures, we established two efficient one-pot sequential coupling routes to 2-amino-3-arylbenzofurans and 2-amino-3-arylnaphtho[2,1-b]furans. Further ring formation (six- and seven-membered rings) with the resulting amine moiety at the C2 position of benzofurans was realized, leading to further expansion of benzofuran-based chemical space.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...