Dative versus electron-sharing bonding in the isoelectronic argon compounds ArR+ (R = CH3, NH2, OH, and F)†
Abstract
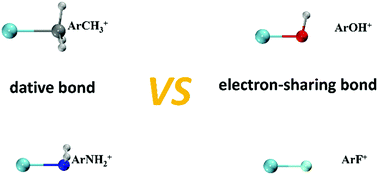
A computational investigation of the isoelectronic ArR+ (R = CH3, NH2, OH, and F) species has been performed. The ground states of all four complexes are determined to be the lowest singlet isomers. A range of state-of-the-art quantum chemistry methods have been utilized to systematically examine the Ar–E bonds (E = C, N, O, and F) in these Ar-containing complexes, which could be categorized to be of covalent type with a gradual increase in the covalent character along C to F. Moreover, the nature of the Ar–E bond roughly depends on the ionization potential difference between the Ar atom and R radical, except for ArOH+. The dative Ar → R+ bond is found for both ArCH3+ and ArNH2+ complexes, whereas the Ar–E bonds in both ArOH+ and ArF+ are better described as electron-sharing Ar+–R bonds.


 Please wait while we load your content...
Please wait while we load your content...