Charge-density induced discrimination of halides with a rigid dinuclear copper(ii) complex†
Abstract
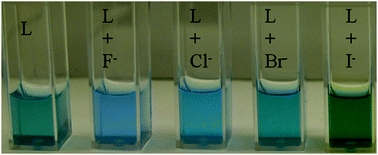
A rigid dinuclear copper(II) complex L based on furan spacers was synthesized and studied for its binding interactions with halides by colorimetric studies, UV-vis titration, and density functional theory (DFT) calculations. Our results from the titration studies demonstrate that L binds each of the halides in the order of fluoride > chloride > bromide > iodide, correlating directly with the charge density of the respective halides. Fully unconstrained DFT geometry optimizations were carried out on both the isolated L and the anion-bound motifs. Binding energies (ΔE) were calculated for each of the optimized geometries, yielding an attractive ΔE value of −92.39, −27.14, −23.16, and −13.37 kcal mol−1 for fluoride, chloride, bromide, and iodide, respectively, which is in accord with our experimental results. The compound has been further investigated for its biocompatibility with HeLa cells, demonstrating an excellent cell viability up to 500 μM concentration.


 Please wait while we load your content...
Please wait while we load your content...
