Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products
Abstract
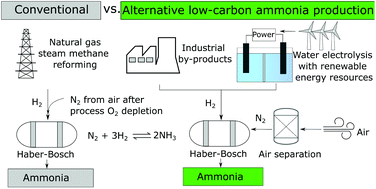
Conventionally, ammonia is produced from natural gas via steam methane reforming (SMR), water-gas shift reaction, and the Haber–Bosch process. The process uses fossil natural gas, which leads to 2.6 metric tons of life cycle greenhouse gas (GHG) emissions per metric ton of ammonia produced. With ammonia being the second most produced chemical in the world, its production accounts for approximately 2% of worldwide fossil energy use and generates over 420 million tons of CO2 annually. To reduce its carbon intensity, ammonia synthesis relying on renewable energy or utilizing by-products from industrial processes is of interest. We conduct a life cycle analysis of conventional and alternative ammonia production pathways by tracking energy use and emissions in all conversion stages, from the primary material and energy resources to the ammonia plant gates. Of all the alternative pathways, obtaining N2 from cryogenic distillation and H2 from low-temperature electrolysis using renewable electricity has the lowest cradle-to-plant-gate GHG emissions, representing a 91% decrease from the conventional SMR pathway.


 Please wait while we load your content...
Please wait while we load your content...
