Humic acid's (HA) role in NDMA formation from daminozide (DMNZD) during ozonation†
Abstract
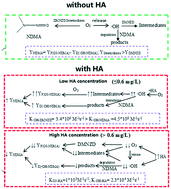
Daminozide (DMNZD) has been found to be an important ozone-reactive N-nitrosodimethylamine (NDMA) precursor; however, the influence of humic acid (HA) on NDMA formation from daminozide during ozonation is unclear. In this study, the impacts of HA on NDMA formation from daminozide at various oxidation conditions (ozone dosages, pH) and other water components (bicarbonate ion (HCO3−), sulfate ion (SO42−)) were investigated. In addition, its implications for water treatment plants are discussed. The results indicated that low levels of HA (≤0.6 mg L−1) facilitated NDMA formation and its molar yield was maximized at 0.1 mg L−1 HA (8.5%), while a high concentration of HA (>0.6 mg L−1) had an inhibition role. The NDMA amount increased with increasing pH and showed a peak at pH 8. With 0.1 mg L−1 HA, NDMA was raised from 3.0% to 23.1% with ozone increasing from 0.5 to 4 mg L−1; HCO3− competed with daminozide for ozone and cut down NDMA formation, whereby 160 μM HCO3− reduced NDMA conversion from 8.5% to 6.5%; however, with the increase in SO42− from 0 to 20 μM, NDMA conversion was augmented from 8.5% to 17.8%. Based on the results of LC/MS/MS and GC/MS analyses, NDMA transformation pathways with/without HA are proposed.


 Please wait while we load your content...
Please wait while we load your content...