Electronic structure influences on the formation of the solid electrolyte interphase†
Abstract
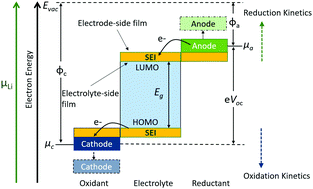
The solid electrolyte interphase (SEI) is critical for lithium-ion batteries (LIBs). Understanding and control over how the SEI is formed is therefore essential to develop LIBs with improved coulombic efficiency, lifetime and capacity. Previous research has focused on how variations in electrolyte chemistry and charging conditions can manipulate the SEI, yet there remains a fundamental lack of understanding surrounding the formation mechanism of the SEI. Specifically, there is minimal research on how the electrode material itself influences the formation of the SEI. Herein, chemically equivalent yet electronically distinct mono-, bi- and multi-layered graphene are used as LIB anodes. The formation of the SEI is probed with respect to kinetics, mechanism, morphology, and composition. SEI films on monolayer graphene contain high amounts of decomposition products from the salt (LiPF6) while on multilayer graphene, the films contain a large proportion of organic products. The basal plane of SEI films contains more organic species in the side close to the electrode than close to the electrolyte because of the intrinsic charge transfer between the solvents and graphitic anodes. We demonstrate that the composition of SEI films is tunable by modifying the reduction capability of the anode relative to the electrolyte where high reduction kinetics promotes LiPF6 decomposition; low reduction kinetics leads to more solvent decomposition and sluggish phase change during SEI deposition. This work provides fundamental insights into the influence of electrode electronic structure on SEI formation, and provides future directions to improve LIBs.


 Please wait while we load your content...
Please wait while we load your content...