Abstract
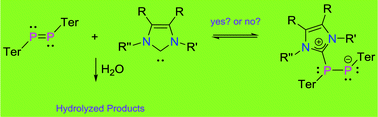
We report the influence of N-heterocyclic carbenes (NHCs) on the hydrolysis of a diphosphene TerP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PTer (1; Ter = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2), a phosphorus-analogue of an alkene. The diphosphene 1 itself is completely inert towards water. However, NHCs have been found to activate 1 towards ready hydrolysis. While sterically less-encumbered NHCs react with 1 affording NHC-adducts which are in equilibrium with 1 in solution, sterically encumbered NHCs do not bind to 1 at all. Interestingly, in both of these situations hydrolysis of the P
PTer (1; Ter = 2,6-Mes2C6H3; Mes = 2,4,6-Me3C6H2), a phosphorus-analogue of an alkene. The diphosphene 1 itself is completely inert towards water. However, NHCs have been found to activate 1 towards ready hydrolysis. While sterically less-encumbered NHCs react with 1 affording NHC-adducts which are in equilibrium with 1 in solution, sterically encumbered NHCs do not bind to 1 at all. Interestingly, in both of these situations hydrolysis of the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P motif proceeds efficiently. At low temperatures, sterically less-encumbered NHCs are catalytic while the sterically encumbered NHCs play a catalytic role at room temperature. To gain insight on this striking influence of NHCs on the hydrolysis of diphosphene detailed low-temperature 31P-NMR studies along with theoretical calculations have been carried out. In addition, systematic hydrolysis studies of all the NHCs used in this study have also been performed.
P motif proceeds efficiently. At low temperatures, sterically less-encumbered NHCs are catalytic while the sterically encumbered NHCs play a catalytic role at room temperature. To gain insight on this striking influence of NHCs on the hydrolysis of diphosphene detailed low-temperature 31P-NMR studies along with theoretical calculations have been carried out. In addition, systematic hydrolysis studies of all the NHCs used in this study have also been performed.


 Please wait while we load your content...
Please wait while we load your content...