Intramolecular (directed) electrophilic C–H borylation
Abstract
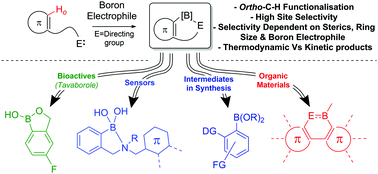
The intramolecular C–H borylation of (hetero)arenes and alkenes using electrophilic boranes is a powerful transition metal free methodology for forming C–B bonds. These C–H borylation reactions are preceded by intermolecular bond (both dative and covalent) formation, with examples proceeding via initial C–B and N–B bond formation dominating this field thus both are discussed in depth herein. Less prevalent intramolecular electrophilic C–H borylation reactions that proceed by intermolecular O–B, S–B and P–B bond formation are also summarised. Mechanistic studies are presented that reveal two mechanisms for C–H borylation, (i) electrophilic aromatic substitution (prevalent with B–X electrophiles); (ii) σ-bond metathesis mediated (prevalent with B–H and B–R electrophiles). To date, intramolecular electrophilic C–H borylation is utilised mainly for accessing boron containing conjugated organic materials, however recent developments, summarized herein alongside early studies, have highlighted the applicability of this methodology for forming synthetically versatile organo-boronate esters and boron containing bioactives. The multitude of synthetic procedures reported for intramolecular electrophilic C–H borylation contain many common features and this enables key requirements for successful C–H borylation and the factors effecting regioselectivity and substrate scope to be identified, discussed and summarized.


 Please wait while we load your content...
Please wait while we load your content...