The atmospheric importance of methylamine additions to Criegee intermediates†
Abstract
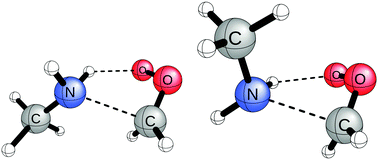
Criegee intermediates are important targets for study in atmospheric chemistry because of their capacity to oxidize airborne species. Among these species, ammonia has received critical attention for its presence in polluted agricultural or industrial areas and its role in forming particulate matter and condensation nuclei. Although methylamine has been given less attention than ammonia, both theoretical and experimental studies have demonstrated that the additional methyl substitution on the ammonia derivatives increases the rate constants for some systems. This suggests that the methylamine addition to Criegee intermediates could be more significant to atmospheric processes. In this work, geometries are optimized at the DF-CCSD(T)/ANO1 level for the methylamine addition reactions to the simplest Criegee intermediate and the anti- and syn-methylated Criegee intermediates. Energies for each stationary point were computed at the CCSD(T)/CBS level with corrections from the CCSDT(Q) method. Rate constants are obtained for each reaction using canonical transition state theory. Although methylamine addition proved to be a more favorable reaction relative to ammonia addition, the significantly lower concentration of atmospheric methylamine limits the prevalence of these reactions, even in the most optimal conditions. It is unlikely that the methylamine addition to Criegee intermediates will contribute significantly to the consumption of Criegee intermediates in the atmosphere.


 Please wait while we load your content...
Please wait while we load your content...
