N-Arylated peptide: troponyl residue influences the structure and conformation of N-troponylated-(di/tri)-peptides†
Abstract
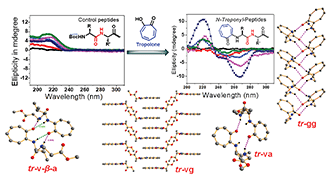
N-arylated peptides as peptoids influence the structural and conformational changes of small peptides that lead to unique foldamers, even in di-/tri-peptides. This report describes the syntheses, structural and conformational studies of rationally designed non-benzenoid N-arylated peptides containing bioactive tropolone molecules such as N-troponylated di-/tri-peptides. This report also demonstrates the role of the troponyl group in influencing the structure and conformation of N-troponylated short peptides (di-/tri-peptides) by single-crystal X-ray data analyses, circular dichroism (CD) studies, and 2D-NMR and DMSO-d61H-NMR titration experiments. The tropolonyl residue has a significant role in the formation of unique supramolecular self-assemblies in dipeptide crystalline solids such as helical/β-sheet/β-turn (antiparallel β-sheet) type secondary structures. The sequence-specific tr-di-/tri-peptides exhibit a characteristic β-strand type of characteristic CD-signal in organic solvents (CH3CN/MeOH). The hydrogen bonding nature of N–H, intra- or intermolecular hydrogen bonding, is also established by DMSO-d61H-NMR titration experiments in the representative tr-di-/tri-peptides. These results explicitly support the role of the troponyl carbonyl group in the formation of secondary structures of peptides, even in di-/tri-peptides. Hence, the N-troponylation of peptides may alter the conformation of peptides to prepare novel peptidomimetics.
- This article is part of the themed collection: Supramolecular & Polymorphism


 Please wait while we load your content...
Please wait while we load your content...