Investigation of the influence of cationic and anionic ions on the oriented dissolution of calcite†
Abstract
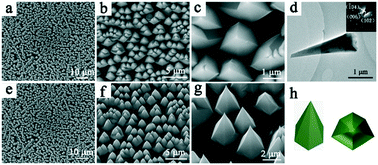
Research on the dissolution or demineralization of minerals can shed light on the biomineralization mechanism. The influence of inorganic ions or organic molecules on the stabilization of specific planes or step edges of crystals or biominerals has attracted significant interest, both in biomineralization and bioinspired crystallization systems. In this work, the influence of cationic and anionic ions on the oriented dissolution of calcite was investigated in detail. The side faces and cross-section geometries of the obtained micropyramids have a strong relationship with the cationic (metal) ions and the pH values of the aqueous solutions. Cations (metal ions) have a more significant influence than anions on the oriented dissolution process of calcite and the metastable side faces and step edges of calcite micropyramids, probably due to the strong interaction between cations and CO32−. Well-aligned c-axis oriented calcite micropyramid arrays with six side faces were fabricated in large scale by the oriented dissolution of calcite {104} planes in an undersaturated aqueous solution of Cu(NO3)2. The metastable step edges ([41![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] and [43
] and [43![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ]) were stabilized by Cu2+ ions in the solution. The pH values of the solutions have also had a key effect on the dissolution kinetics, dissolution rates and metastable side faces of the obtained one-dimensional microstructures of calcite.
]) were stabilized by Cu2+ ions in the solution. The pH values of the solutions have also had a key effect on the dissolution kinetics, dissolution rates and metastable side faces of the obtained one-dimensional microstructures of calcite.


 Please wait while we load your content...
Please wait while we load your content...